Wind power
Wind power is the conversion of wind energy into more useful forms, such as electricity, using wind turbines.
At the end of 2006, worldwide capacity of wind-powered generators was
73.9 gigawatts; although it currently produces just over 1% of
world-wide electricity use, it accounts for approximately
20% of
electricity use in Denmark, 9% in Spain, and 7% in Germany.Globally,
wind power generation more than quadrupled between 2000 and 2006.
Most modern wind power is generated in the form of electricity by
converting the rotation of turbine blades into electrical current by
means of an electrical generator.
In windmills (a much older technology), wind energy is used to turn
mechanical machinery to do physical work, such as crushing grain or
pumping water.
Wind power is used in large scale wind farms for national electrical
grids as well as in small individual turbines for providing electricity
to rural residences or grid-isolated locations.
Wind energy is plentiful, renewable, widely distributed, clean, and
reduces toxic atmospheric and greenhouse gas emissions if used to
replace fossil-fuel-derived electricity.
The average output of one megawatt of wind power is equivalent to the
average electricity consumption of about
250 American households.
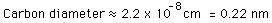
 Upon entering a laboratory, you see an experiment. How do you know which class it belongs to?
Upon entering a laboratory, you see an experiment. How do you know which class it belongs to?